Valley Fever Solutions
Bringing New Hope to an Orphan Disease


Cocci (Valley Fever) is an orphan disease,
infecting 150 K humans/yr
Every year, about 150 people die from Valley Fever (coccidioidomycosis). About 2,000 are hospitalized, with hospital costs exceeding $200M/yr in Arizona and California alone. About 50,000 people a year are symptomatic, many of them seriously so. Not all of these cases are diagnosed, but we know that over 10,000 cases are reported each year in Arizona (90 per 100,000 in 2013). A yearly average of about 20,000 people have severe disease that would benefit from better treatment. Several thousand have no practical solution, as the available drugs do not help them, or the side effects of current drugs are too onerous.
Lost work time and suffering are additional costs. A greater impact is seen for some minorities. Valley Fever also infects 160K pets, especially dogs.
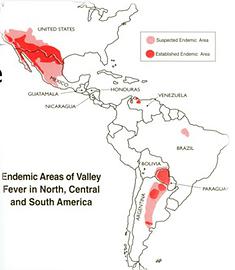
The Endemic Area is concentrated
60% of all US infections occur in the 100 miles between Phoenix and Tucson. Another 10% is clustered in the southern Central Valley of California, particularly Bakersfield and Kern County. Most of the rest is clustered roughly along the US border with Mexico. This means that efforts to treat the disease can be very focused, requiring a rather small sales force. The bulk of known cases are in the US.
Under diagnosed
The sensitivity of tests for early detection could be greatly improved. We have identified potential markers that could form the basis for a companion diagnostic. We welcome support for this effort.
Current treatments have shortcomings
Current standard-of-care treatments only arrest the cocci infection, but do not cure. Particularly among the very sick, 60% of patients are unresponsive to current therapies. The currently available treatments have many side effects. Reliable animal models, that are highly predictive of human effects for existing drugs for Valley Fever, show NikZ is fungicidal. From this and other studies, we observe at most minimal side effects. This is very promising for further study of NikZ, and we hope for rapid approval.